This website is intended for use by US healthcare professionals only.
Be in your
element
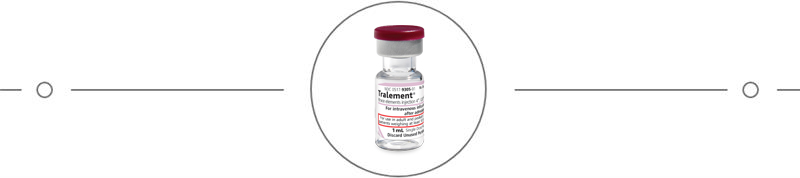
Focus on what you do best with Tralement (trace elements injection 4*, USP) and Multrys (trace elements injection 4*, USP) formulations for parenteral nutrition (PN).
Tralement and Multrys are the first FDA-approved multi-trace element formulations.1
Trace elements (TEs) are essential. Per American Society for Parenteral and Enteral Nutrition (ASPEN) clinical recommendations, patients should receive a daily dose of TEs as part of their PN order.2

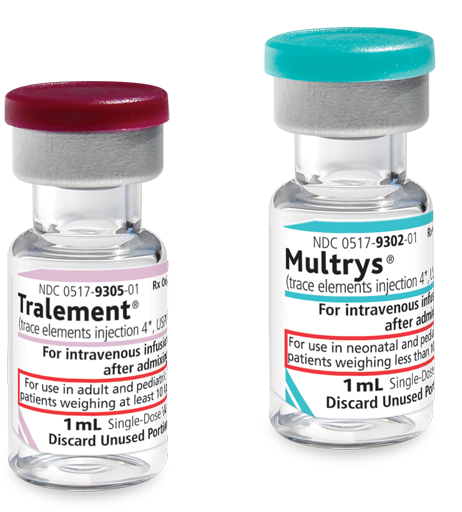
Tralement is indicated for adult and pediatric patients weighing at least 10 kg as a source of zinc, copper, manganese, and selenium for parenteral nutrition when oral or enteral nutrition is not possible, insufficient, or contraindicated3
Multrys is indicated for neonatal and pediatric patients weighing less than 10 kg as a source of zinc, copper, manganese, and selenium for parenteral nutrition when oral or enteral nutrition is not possible, insufficient, or contraindicated4

Actor Portrayal
Tralement and Multrys are the first FDA-approved multi-trace element formulations.1
Trace elements (TEs) are essential. Per American Society for Parenteral and Enteral Nutrition (ASPEN) clinical recommendations, patients should receive a daily dose of TEs as part of their PN order.2


Tralement is indicated for adult and pediatric patients weighing at least 10 kg as a source of zinc, copper, manganese, and selenium for parenteral nutrition when oral or enteral nutrition is not possible, insufficient, or contraindicated3
Multrys is indicated for neonatal and pediatric patients weighing less than 10 kg as a source of zinc, copper, manganese, and selenium for parenteral nutrition when oral or enteral nutrition is not possible, insufficient, or contraindicated4
Our Commitment to Parenteral Nutrition (PN)
American Regent is committed to supporting the needs of clinicians and their patients. View highlights from our 30+ years of partnering with the PN community.
NEED ANSWERS?

View Frequently Asked Questions (FAQs)
Get answers to common inquiries clinicians ask regarding Tralement and Multrys as an additive to PN, including formulations, storage, and more.
References:
- Orange book: approved drug products with therapeutic equivalence evaluations: product details for NDA 209376. US Food & Drug Administration. Multrys and Tralement: Accessed May 13, 2025. https://www.accessdata.fda.gov/scripts/cder/ob/results_product.cfm?Appl_Type=N&Appl_No=209376.
- American Society for Parenteral and Enteral Nutrition. Appropriate dosing for parenteral nutrition: ASPEN Recommendations. Published November 17, 2020. Accessed May 13, 2025. https://nutritioncare.org/wp-content/uploads/2024/12/Appropriate-Dosing-for-PN.pdf.
- Tralement (trace elements injection 4*, USP) Package insert. Shirley, NY: American Regent, Inc.
- Multrys (trace elements injection 4*, USP) Package insert. Shirley, NY: American Regent, Inc.
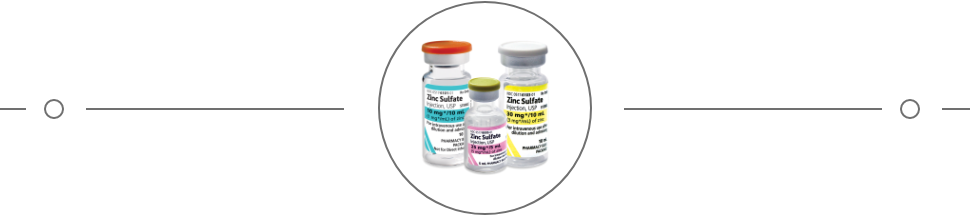
- Zinc Sulfate Injection Package Insert. Shirley, NY: American Regent, Inc.
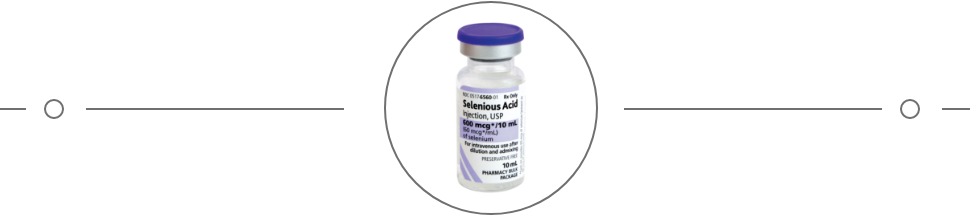
- Selenious Acid Injection Package Insert. Shirley, NY: American Regent, Inc.
- Soule LM; US Food & Drug Administration. NDA 209377. Published July 18, 2019. Accessed May 13, 2025. https://www.accessdata.fda.gov/drugsatfda_docs/appletter/2019/209377Orig1s000ltr.pdf.
- Nikhar B; US Food & Drug Administration. NDA 209379. Published April 30, 2019. Accessed May 13, 2025. https://www.accessdata.fda.gov/drugsatfda_docs/nda/2019/209379Orig1s000Approv.pdf.
- Vanek VW, Borum P, Buchman A, et al; Novel Nutrient Task Force; Parenteral Multi-Vitamin and Multi-Trace Element Working Group; American Society for Parenteral and Enteral Nutrition (A.S.P.E.N.) Board of Directors. A.S.P.E.N. position paper: recommendations for changes in commercially available parenteral multivitamin and multi-trace element products. Nutr Clin Pract. 2012;27(4):440-491.
IMPORTANT SAFETY INFORMATION
Tralement (trace elements injection 4*, USP)
CONTRAINDICATIONS
Tralement is contraindicated in patients with hypersensitivity to zinc or copper.
WARNINGS AND PRECAUTIONS
Pulmonary Embolism due to Pulmonary Vascular Precipitates: Pulmonary vascular precipitates causing pulmonary vascular emboli and pulmonary distress have been reported in patients receiving parenteral nutrition. If signs of pulmonary distress occur, stop the infusion and initiate a medical evaluation.
Vein Damage and Thrombosis: Tralement must be prepared and used as an admixture in parenteral nutrition solution. It is not for direct intravenous infusion. In addition, consider the osmolarity of the final parenteral nutrition solution in determining peripheral versus central administration. Solutions with osmolarity of 900 mOsmol/L or more must be infused through a central catheter. The primary complication of peripheral access is venous thrombophlebitis.
Neurologic Toxicity with Manganese: Monitor patients receiving long-term parenteral nutrition solutions containing Tralement for neurologic signs and symptoms, and routinely monitor whole blood manganese concentrations and liver function tests. Discontinue Tralement and consider brain magnetic resonance imaging (MRI) if toxicity suspected.
Hepatic Accumulation of Copper and Manganese: If a patient develops signs or symptoms of hepatic or biliary dysfunction during the use of Tralement, obtain serum concentrations of copper and ceruloplasmin as well as manganese whole blood concentrations. Consider using individual trace element products in patients with hepatic and/or biliary dysfunction.
Aluminum Toxicity: Tralement contains aluminum that may be toxic. Increased risk in patients with renal impairment. Preterm infants, including preterm neonates, are particularly at risk.
Monitoring and Laboratory Tests: Monitor blood zinc, copper, manganese, and selenium concentrations, fluid and electrolyte status, serum osmolarity, blood glucose, liver and kidney function, blood count, and coagulation parameters.
Hypersensitivity Reactions with Zinc and Copper: If hypersensitivity reactions occur, discontinue Tralement and initiate appropriate medical treatment.
ADVERSE REACTIONS
The following adverse reactions were identified in clinical studies or post-marketing reports. Given that some of these reactions were reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Adverse reactions with other components of parenteral nutrition solutions:
- Pulmonary embolism due to pulmonary vascular precipitates
- Vein damage and thrombosis
- Aluminum toxicity
Adverse reactions with the use of trace elements administered parenterally or by other routes of administration:
- Neurologic toxicity with manganese
- Hepatic accumulation of copper and manganese
- Hypersensitivity reactions with zinc and copper
USE IN SPECIFIC POPULATIONS
Pregnancy - Risk Summary - Deficiency of trace elements may result in adverse pregnancy and fetal outcomes.
Lactation - Risk Summary - Zinc, copper, manganese, and selenium are present in human milk. The developmental and health benefits of breastfeeding should be considered, along with the mother’s clinical need for Tralement and any potential adverse effects on the breastfed infant from Tralement or from the underlying maternal condition.
Pediatric Use - Refer to Full Prescribing Information for dosing. Do not supplement Tralement with additional manganese. Tralement is not approved for use in pediatric patients weighing less than 10 kg because the product does not provide an adequate dosage of zinc, copper, or selenium to meet the needs of this subpopulation and exceeds the recommended dosage of manganese.
Hepatic Impairment - Hepatic accumulation of copper and manganese have been reported with long-term administration in parenteral nutrition. For patients with cholestasis, biliary dysfunction, or cirrhosis, monitor hepatic and biliary function during long-term administration of Tralement.
OVERDOSAGE
There are reports on overdosage in the literature for the individual trace elements. Management of overdosage is supportive care based on presenting signs and symptoms.
Tralement is recommended only for patients who require supplementation with all four of the individual trace elements (ie, zinc, copper, manganese, and selenium).
INDICATIONS AND USAGE
Tralement is indicated in adult and pediatric patients weighing at least 10 kg as a source of zinc, copper, manganese, and selenium for parenteral nutrition when oral or enteral nutrition is not possible, insufficient, or contraindicated.
For additional safety information, please see Full Prescribing Information.
You are encouraged to report Adverse Drug Events (ADEs) to American Regent, Inc. at 1-800-734-9236, or to the FDA by visiting fda.gov/safety/medwatch or calling 1-800-FDA-1088.
REF-1535 7/2024
Multrys (trace elements injection 4*, USP)
CONTRAINDICATIONS
Contraindicated in patients with hypersensitivity to zinc or copper.
WARNINGS AND PRECAUTIONS
Pulmonary Embolism due to Pulmonary Vascular Precipitates: Pulmonary vascular precipitates causing pulmonary vascular emboli and pulmonary distress have been reported in patients receiving parenteral nutrition. If signs of pulmonary distress occur, stop the parenteral nutrition infusion and initiate a medical evaluation.
Vein Damage and Thrombosis: Multrys must be prepared and used as an admixture in parenteral nutrition solution. It is not for direct intravenous infusion. In addition, consider the osmolarity of the final parenteral nutrition solution in determining peripheral versus central administration. Solution with an osmolarity of 900 mOsmol/L or greater must be infused through a central catheter. The infusion of hypertonic nutrient solution into a peripheral vein may result in vein irritation, vein damage, and/or thrombosis.
Neurologic Toxicity with Manganese: Monitor for clinical signs and symptoms of neurotoxicity, whole blood manganese concentrations, and liver function tests. Discontinue Multrys and consider brain magnetic resonance imaging (MRI) if toxicity is suspected. Monitor patients for cholestasis or other biliary liver disease.
Hepatic Accumulation of Copper and Manganese: If a patient develops signs or symptoms of hepatobiliary disease during the use of Multrys, obtain serum concentrations of copper and ceruloplasmin as well as manganese whole blood concentrations; consider using individual trace element products in these patients.
Aluminum Toxicity: Multrys contains aluminum that may be toxic. Patients with renal impairment and preterm infants, including preterm neonates, are particularly at risk.
Monitoring and Laboratory Tests: Monitor blood zinc, copper, and selenium serum concentrations, whole blood manganese concentration, fluid and electrolyte status, serum osmolarity, blood glucose, liver and kidney function, blood count, and coagulation parameters.
Hypersensitivity Reactions with Zinc and Copper: If hypersensitivity reactions occur, discontinue and initiate appropriate medical treatment.
ADVERSE REACTIONS
The following adverse reactions were identified in clinical studies or post-marketing reports. Given that some of these reactions were reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Adverse reactions with other components of parenteral nutrition solutions:
- Pulmonary embolism due to pulmonary vascular precipitates
- Vein damage and thrombosis
- Aluminum toxicity
Adverse reactions with the use of trace elements administered parenterally or by other routes of administration:
- Neurologic toxicity with manganese
- Hepatic accumulation of copper and manganese
- Hypersensitivity reactions with zinc and copper
USE IN SPECIFIC POPULATIONS
Hepatic Impairment - Hepatic accumulation of copper and manganese have been reported with long-term administration in parenteral nutrition. For patients with cholestasis, biliary dysfunction, or cirrhosis, monitor hepatic and biliary function during long-term administration of Multrys.
OVERDOSAGE
There are reports on overdosage in the literature for the individual trace elements. Management of overdosage is supportive care based on presenting signs and symptoms.
INDICATIONS AND USAGE
Multrys is a combination of trace elements (zinc sulfate, cupric sulfate, manganese sulfate, and selenious acid) indicated in neonatal and pediatric patients weighing less than 10 kg as a source of zinc, copper, manganese, and selenium for parenteral nutrition when oral or enteral nutrition is not possible, insufficient, or contraindicated.
For additional safety information, please see Full Prescribing Information.
You are encouraged to report Adverse Drug Events to American Regent, Inc. at 1-800-734-9236, or to the FDA by visiting fda.gov/safety/medwatch or by calling 1-800-FDA-1088.
REF-1826 7/2024